We use cookies on this website to enhance your user experience. Please read our cookie policy for additional information and a complete overview. We will only use cookies if you consent by clicking on “Accept all cookies”. You can always manage your preferences via the settings of your browser. Please note that certain media will only be available if you have accepted the applicable cookies.
A 10-year old boy with refractory focal epilepsy
Medical history and disease course
The patient had been experiencing seizures since the age of four. Initially they presented as a tonic extension of the left arm and leg, which evolved to a bilateral tonic-clonic seizure. At 8 years, a new type of seizure presented with paresthesia in the left hand followed by sudden falls. Due to refractoriness to several anti-epileptic drugs, he was referred to the reference center for epilepsy surgery to determine if resective epilepsy surgery would be feasible.
Preoperative workup
Brain MRI with optimal epilepsy protocol was normal. The patient had several seizures during the long-term video-EEG-monitoring session that were described as habitual seizures. One example is shown below in fig. 1, where the seizure started with extension of the left arm followed by pedalling of both legs. There was no postictal deficit. The EEG shows a generalized decrement preceded by ictal fast rhythmic discharges that are most prominent over the right frontal electrode (F4). Interictal discharges were localized in the right central region. Based on seizure semiology and ictal EEG, it was proposed that the epileptogenic region was most probably localized extratemporally in the right hemisphere, possibly in the right frontal lobe.
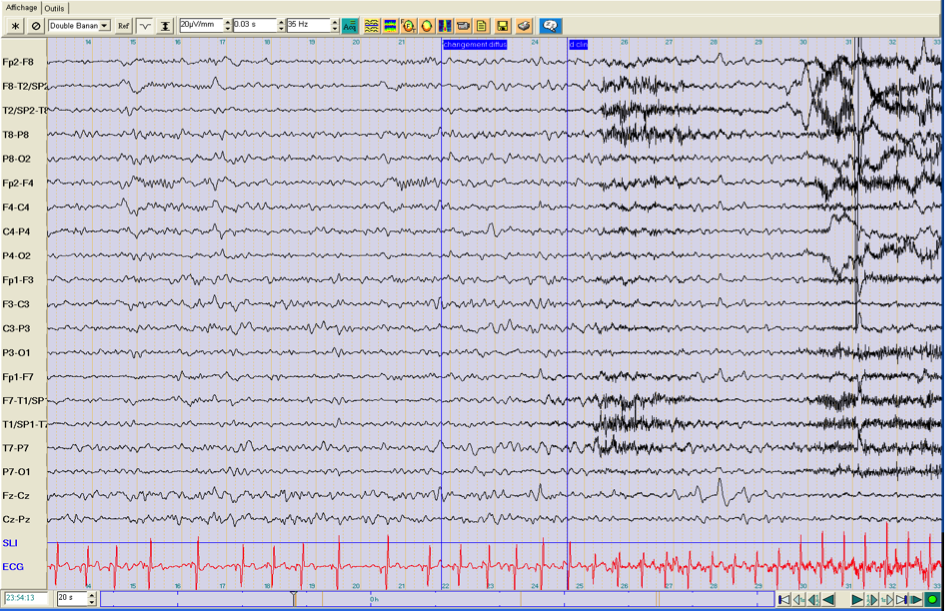
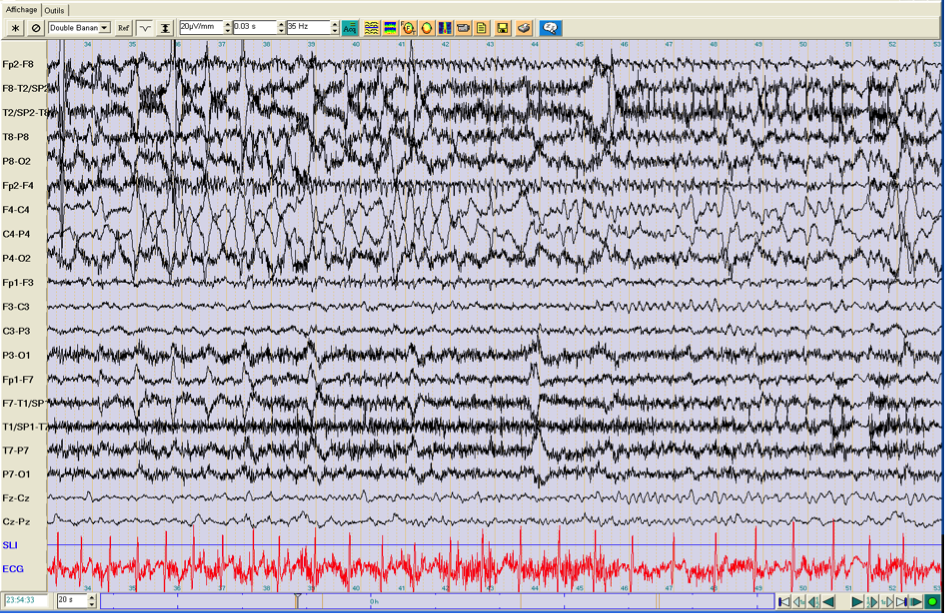 Fig. 1: ictal EEG of a seizure (top = onset, bottom = continued) that started with the extension of the right arm, followed by pedaling movements of both legs. There was no secondary generalization and no postictal deficit.
Fig. 1: ictal EEG of a seizure (top = onset, bottom = continued) that started with the extension of the right arm, followed by pedaling movements of both legs. There was no secondary generalization and no postictal deficit.
Due to the absence of an MRI-lesion and ill-defined ictal onset, additional investigations were planned. Interictal FDG-PET showed hypometabolism in the right insular and opercular region. Electrical source imaging of interictal spikes from the long-term video-EEG-monitoring was performed. It showed a dominant spike cluster with the maximum at T8. Source imaging localized this spike to the right insular and parietal operculum region, as shown in fig. 2.
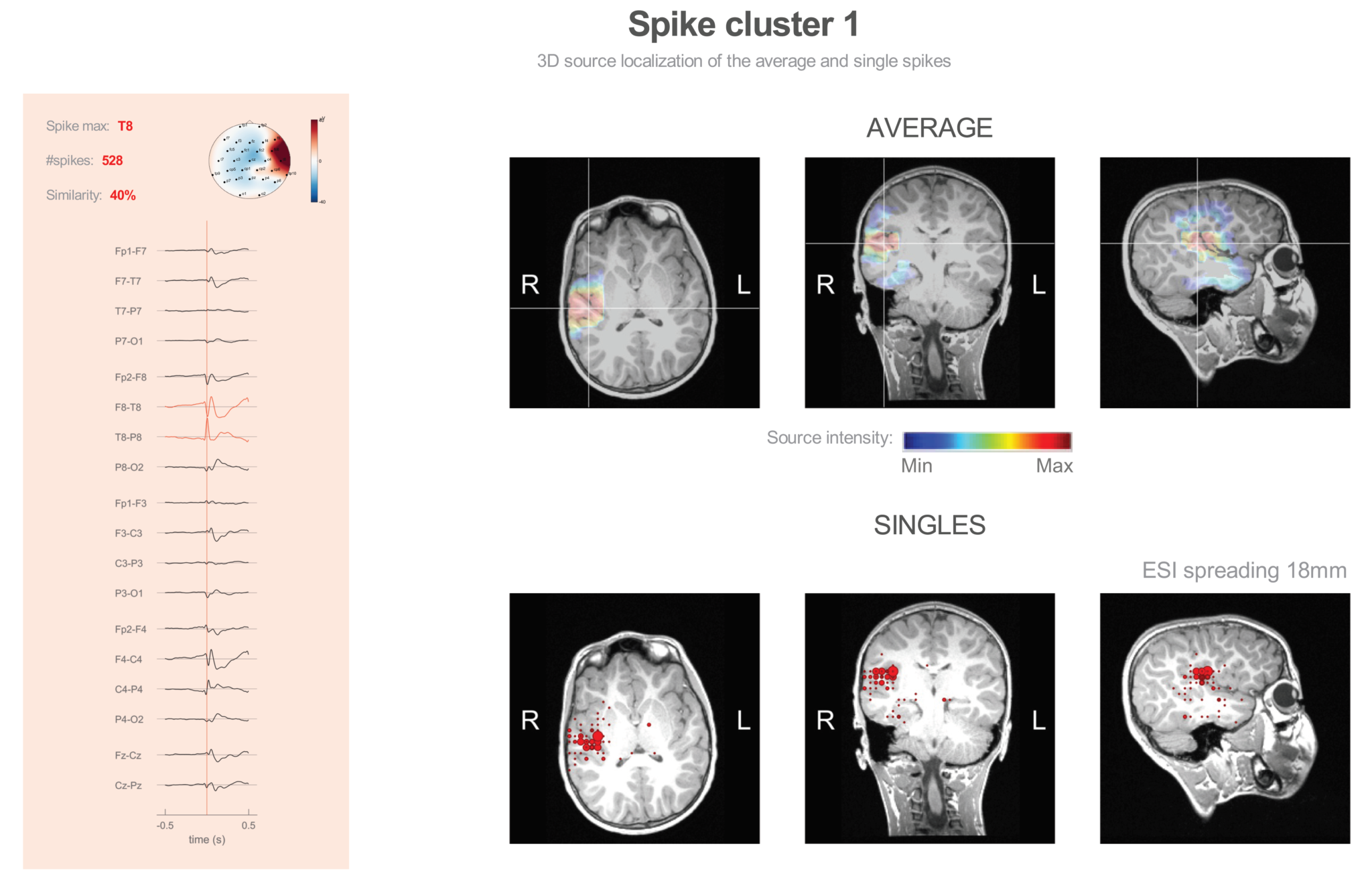
Fig. 2: Results showing ESI of spikes detected during long-term video-EEG monitoring. The distributed source model of the average spike localizes to the right insular – parietal operculum region.
Invasive video-EEG-monitoring
Based on this information, an invasive video-EEG-monitoring session was planned, with a large grid over the right fronto-temporo-parietal region as shown in fig. 3. Interictal spikes were recorded over the D6-F7 region. Several habitual seizures were recorded, of which one is shown in fig. 4. It starts with diffuse low voltage fast activity. Interictal discharges were found in contacts overlying the right opercular – inferior frontal region.
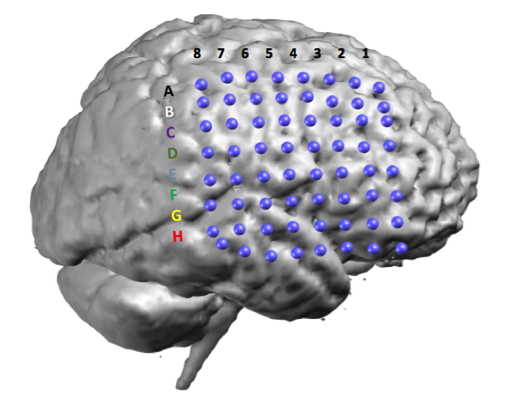
Fig. 3: placement of a large grid over the right fronto-temporo-parietal region for invasive video-EEG-monitoring.
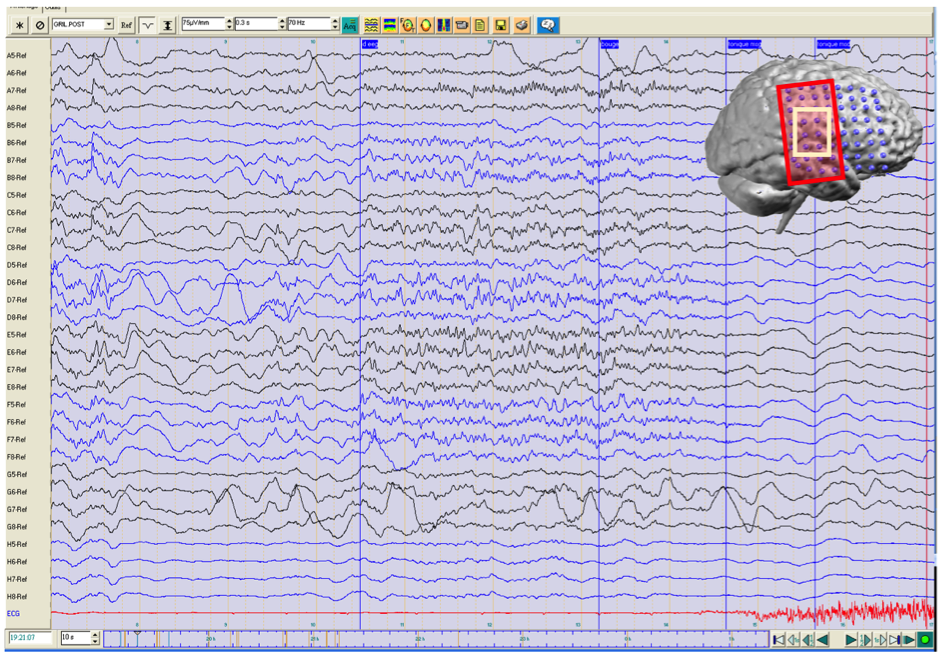
Fig. 4: invasive EEG during a habitual seizure. The first sign of seizure activity is low voltage fast activity over a large region (red rectangle). Interictal EEG discharges appear over a more limited area (light yellow rectangle).
Resective epilepsy surgery
Based on the findings during the invasive video-EEG-monitoring session, the right parietal operculum was resected. A postoperative MRI is depicted in fig. 5. The patient has since then been seizure free for > 4 years. Histopathology: dysplasia type IIa.
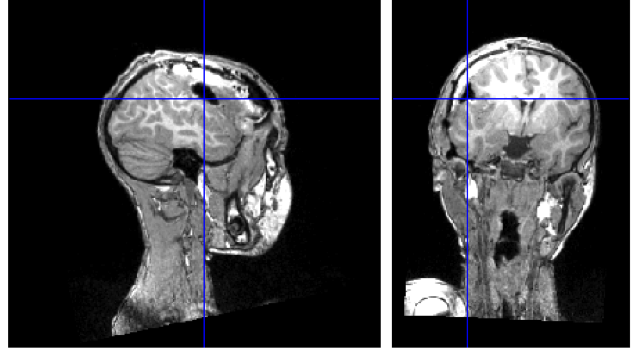
Fig. 5: postoperative MRI after resection of the right parietal operculum.
Interpretation
The current case nicely illustrates the added value of using electrical source imaging in the preoperative work-up. MRI-negative refractory epilepsies are the most difficult surgical candidates. Although habitual seizures were recorded during the video-EEG-monitoring session, the combination of the ictal EEG pattern with seizure semiology did not allow the definition of a good hypothesis. Additional information came from electrical source imaging and FDG-PET allowing targeted implantation of subdural electrodes for invasive video-EEG-monitoring and later respective surgery. Findings from the intracranial EEG were concordant with the results of non-invasive electric source localization. Postoperative seizure control still persists, providing additional evidence that the epileptogenic zone was indeed resected.
Acknowledgment
We would like to thank prof. M. Seeck from Geneva University Hospital for providing the clinical and imaging data of this case.
